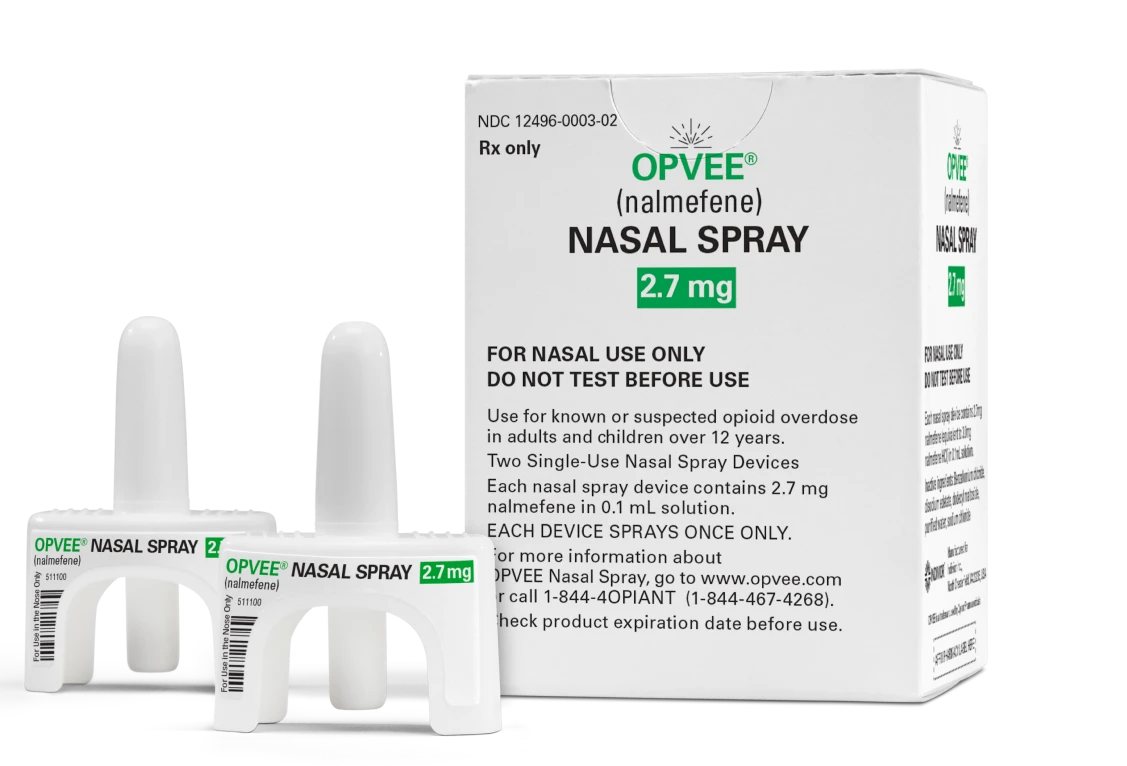
IMPORTANT SAFETY INFORMATION
CONTRAINDICATION: Do not use in patients who are allergic to nalmefene or any of the other ingredients.
WARNINGS AND PRECAUTIONS
Risk of Recurrent Respiratory and Central Nervous System Depression: While the duration of action of nalmefene is as long as most opioids, a recurrence of slowed breathing (respiratory depression) is possible after treatment with OPVEE®. Watch patients and give repeat doses of OPVEE® using a new device, as necessary, while awaiting emergency medical assistance.
Risk of Limited Efficacy with Partial Agonists or Mixed Agonist/Antagonists: Improvement in respiratory depression caused by medicines such as buprenorphine and pentazocine may not be complete. Repeat doses of OPVEE® may be required.
Precipitation of Severe Opioid Withdrawal: Use in patients who are opioid dependent may cause symptoms of opioid withdrawal like body aches, fever, sweating, runny nose, sneezing, goose bumps, yawning, weakness, shivering or trembling, nervousness, restlessness or irritability, diarrhea, nausea or vomiting, abdominal cramps, increased blood pressure, and rapid heart rate. Some patients may become aggressive when an opioid overdose is treated.
Abrupt postoperative reversal of opioid depression may result in adverse cardiovascular (CV) effects. These events have primarily occurred in patients with preexisting CV disorders or who received other drugs with similar adverse CV effects. Monitor these patients closely in an appropriate healthcare setting.
In newborns, opioid withdrawal may be life-threatening if not recognized and properly treated and may also include convulsions, excessive crying, and hyperactive reflexes. Monitor for symptoms of opioid withdrawal.
Risk of Opioid Overdose from Attempts to Overcome the Blockade: Taking large or repeated doses of opioids, such as heroin or prescription pain pills to overcome blockade, may lead to opioid intoxication and death.
ADVERSE REACTIONS: Most common adverse reactions (incidence at least 2%) are nasal discomfort, headache, nausea, dizziness, hot flush, vomiting, anxiety, fatigue, nasal congestion, throat irritation, pain in the nose, decreased appetite, changes in sense of taste, skin redness, and increased sweating.
To report a pregnancy or side effects associated with taking OPVEE® or any safety related information, product complaint, request for medical information, or product query, please contact or 1-877-782-6966. You are encouraged to report negative side effects of drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see full Prescribing Information.